As the 2024-2025 flu season approaches, it’s important for medical practices to be up-to-date on the proper coding and billing procedures for influenza vaccinations. Administering the flu shot is a crucial part of preventive care, but navigating the complexities of medical billing for these services can be challenging. Medical billing and coding outsourcing to an expert is a practical way to ensure accurate documentation for the influenza vaccine, complimenting patient care efforts and supporting proper claim submission and reimbursement. This post provides an overview of the coding and billing requirements for influenza vaccinations in 2024-2025.


Ensure proper submission of influenza vaccination claims and optimize revenue.
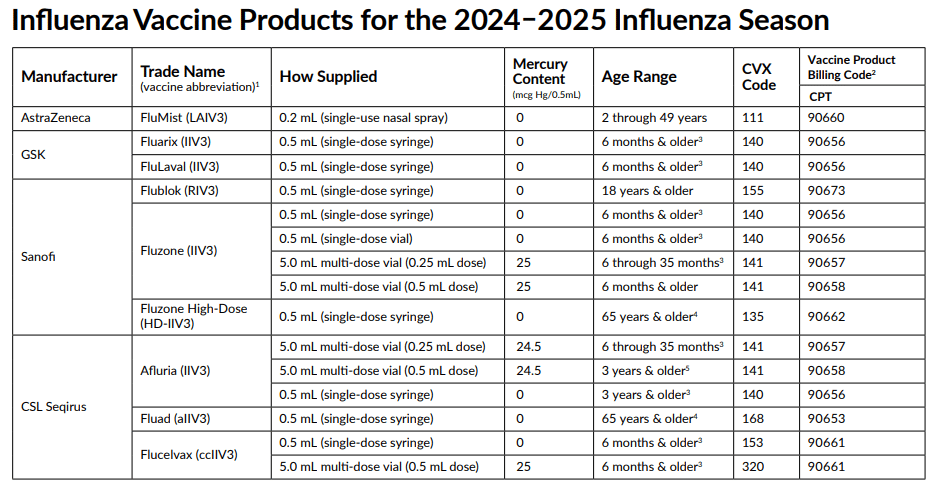
2024–2025 Seasonal Influenza Vaccines and Their CPT Codes
Here are some key points about the influenza vaccines for the upcoming flu season:
- All 2024–2025 seasonal influenza vaccines are trivalent. IIV = egg-based inactivated influenza vaccine (injectable).
- In addition to the vaccine product code, an administration code should always be reported. The CDC explains, “Where only a single vaccine exists, the CPT code is mapped to that vaccine specific CVX code. However, in many cases, a single CPT code could be used with multiple vaccines utilizing different CVX codes. In this situation, the CPT code is mapped to the “unspecified formulation” CVX code because it is not possible to infer a specific vaccine formulation from the CPT code alone”.
- Payers may have unique policies and documentation requirements that need to be included when submitting claims for influenza vaccinations.
Here is a list of the influenza vaccines for the upcoming season along with their CPT codes and CVX codes:
90660 Influenza virus vaccine, trivalent, live (LAIV3), for intranasal use
90656 Influenza virus vaccine, trivalent (IIV3), split virus, preservative free, 0.5 mL dosage, for intramuscular use, 6 months & older
90673 Influenza virus vaccine, trivalent (RIV3), derived from recombinant DNA, hemagglutinin (HA) protein only, preservative and antibiotic free, for intramuscular use 0.5 mL (single-dose syringe)
90656 Influenza virus vaccine, trivalent (IIV3), split virus, preservative free, 0.5 mL dosage (single-dose syringe) for intramuscular use
90656 … 0.5 mL (single-dose vial) for intramuscular use
90657 Influenza virus vaccine, trivalent (IIV3), split virus, 0.25 mL dosage, for intramuscular use
90658 Influenza virus vaccine, trivalent (IIV3), split virus, multi-dose vial (0.5 mL dose) for intramuscular use
90662 Trivalent, inactivated, split-virus influenza vaccine 0.5 mL (single-dose syringe) indicated for use in older adults
90653 Influenza vaccine, inactivated (IIV), subunit, adjuvanted, for intramuscular use, 0.5 mL (single-dose syringe)
90661 Influenza virus vaccine, trivalent (ccIIV3), derived from cell cultures, subunit, preservative and antibiotic free, 0.5 mL dosage, for intramuscular use
ICD-10 Coding for Immunizations
ICD-10 code Z23 is reported for vaccine-related encounters for all vaccines given within the encounter. Z23 is the ICD-10 code that identifies an encounter for immunization(s). If the influenza vaccination is provided in response to a potential exposure (e.g., Td vaccine administered as part of wound care), the ICD-10 code describing the underlying condition should be reported as the primary diagnosis, with Z23 (Encounter for immunization) used as the secondary diagnosis code.
Billing Guidelines
Here are some key billing guidelines from the 2024 National Adult and Influenza Immunization Summit:
- Influenza vaccinations can be billed on the same date of service as an E/M visit or a preventive care appointment. In these cases, providers should report the appropriate CPT vaccine administration code alongside the relevant CPT or HCPCS code for the vaccine product. These codes should be linked to the applicable ICD-10 diagnosis code to demonstrate the medical necessity for administering the influenza vaccine.
- When an E/M service, other than a preventive care visit, is provided on the same date as a prophylactic immunization, modifier 25 may be appended to the E/M service code. This indicates that the E/M service was a significant and separately identifiable element of the encounter, distinct from the work involved in administering the vaccine. Conversely, if there is no E/M or preventive visit or if the sole purpose of the encounter was to administer immunizations, then the use of modifier 25 is not required.
- The actual administration of the vaccine and the vaccine product should be billed separately.
- For patients aged 18 years and younger, providers may report CPT codes that include counseling by a physician or other qualified healthcare professional. This is appropriate if the provider delivers face-to-face counseling during the same visit where vaccines are administered. For patients over the age of 18, it may be appropriate to also report an E/M visit code in addition to the vaccine administration code, if the counseling provided by the physician or qualified healthcare provider exceeds the typical services included in the vaccine administration.
- Payers have specific timelines for submitting claims. Claims filed after this period will be denied. Importantly, claims must accurately reflect the actual date the service was provided.
Leveraging medical billing services can help you optimize reimbursement for administering influenza vaccinations. In addition to maintaining current knowledge of the season’s available vaccine products and associated codes, experienced coders at medical billing companies are well-versed in payer policies and documentation requirements. This expertise can help ensure claims are submitted accurately and in compliance with payer guidelines.

Our medical billing and coding services can maximize your reimbursement for flu shots this season!